
All iLive content is medically reviewed or fact checked to ensure as much factual accuracy as possible.
We have strict sourcing guidelines and only link to reputable media sites, academic research institutions and, whenever possible, medically peer reviewed studies. Note that the numbers in parentheses ([1], [2], etc.) are clickable links to these studies.
If you feel that any of our content is inaccurate, out-of-date, or otherwise questionable, please select it and press Ctrl + Enter.
Why Photoreceptor Cell Survival Does Not Reach 100%: Key Mechanisms Elucidated
Last reviewed: 09.08.2025
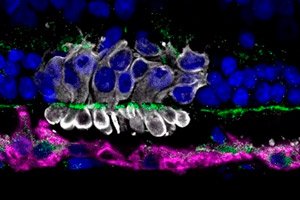
Scientists from the University of Pennsylvania, led by Raghavi Sudharsan, have discovered why about 70% of transplanted photosensory progenitor cells (PRPCs) from induced pluripotent stem cells die within the first few days after transplantation into the retina. Their work, published in Stem Cell Research & Therapy, points to metabolic stress in donor cells as the main culprit behind the early loss.
Prerequisites
Transplantation of PRPCs is considered a promising approach for progressive degenerative diseases of the retina (retinitis pigmentosa, macular degeneration). However, the low survival rate of donor cells limits the effectiveness of therapy. Until now, the main focus was on suppressing the immune response, but even with extensive immunosuppression, losses remained catastrophic.
Design and methods
Models:
Healthy dogs and retinitis pigmentosa model dogs received subretinal injections of fluorescently labeled PRPCs.
Survival Estimate:
Fluorescein angiography and optical coherence tomography (OCT) recorded the volume of transplanted cells on the first day, on the 3rd and on the 7th day.
Single cell transcriptomics (scRNA-seq):
PRPCs were isolated from retinal sites on day 3 and the expression of genes related to metabolism and apoptosis was analyzed.
Immunohistochemistry:
Oxidative stress markers (4-HNE), mitochondrial status (Tom20), and microglial activation (Iba1) were assessed in the transplant area.
Key Results
- Massive cell loss: approximately 70% of PRPCs disappeared by day 7 in both healthy and degenerating retinas despite immunosuppression.
- Metabolic stress: scRNA-seq revealed a dramatic decrease in the expression of oxidative phosphorylation genes (CYCS, COX4I1) and an increase in the transcription of apoptotic markers (BAX, CASP3).
- Mitochondrial dysfunction: Immunohistochemistry showed fragmentation and loss of Tom20 labeling in transplanted PRPCs, and elevated 4-HNE levels indicated oxidative damage.
- Role of microglia: In the transplant area, Iba1⁺ microglial cell activity increased in response to PRPCs death, which could exacerbate local inflammation and contribute to further losses.
Implications for cell therapies
These findings change the paradigm: to increase the engraftment of PRPCs, it is necessary not only to suppress the immune response, but also to support the energy metabolism of donor cells. Possible interventions:
- Preliminary "metabolic pre-training" of PRPCs under normal culture conditions at the edge of substress loading to enhance their mitochondrial resilience.
- Cocktails of mitochondrial stabilizers (coenzyme Q₁₀, carnitine) during and immediately after transplantation.
- Modulation of the local retinal microenvironment: delivery of antioxidants or mitochondrial protectors to the transplantation area.
Practical conclusions and prospects
- Metabolic preconditioning: conditioning PRPCs under mild metabolic stress conditions prior to transplantation to enhance their resilience.
- Scaffold delivery: the use of biodegradable matrices that ensure the gradual transition of donor cells from a rich culture medium into the retinal environment.
- Nutritional support: delivery of antioxidants or mitochondrial respiration substrates in conjunction with PRPCs.
“We have long been fighting only the immune barrier, but now it is clear that without solving the problem of metabolic shock, transplants are doomed to early death,” concludes Raghavi Sudharsan.
The work, supported by the National Eye Institute, paves the way for more viable cell therapies to restore vision in patients with degenerative retinal diseases.
