
All iLive content is medically reviewed or fact checked to ensure as much factual accuracy as possible.
We have strict sourcing guidelines and only link to reputable media sites, academic research institutions and, whenever possible, medically peer reviewed studies. Note that the numbers in parentheses ([1], [2], etc.) are clickable links to these studies.
If you feel that any of our content is inaccurate, out-of-date, or otherwise questionable, please select it and press Ctrl + Enter.
"Smart" hydrogel for diabetic wounds: antiseptic, anti-inflammatory, and help with sugar
Last reviewed: 09.08.2025
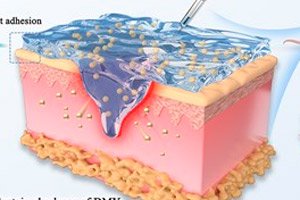 ">
">Diabetic wounds, especially foot ulcers, heal slowly: infections (MRSA and others), excess reactive oxygen species (ROS), prolonged inflammation, poor vascular growth, and chronically high sugar levels prevent the skin from recovering. There is no simple “one-button” solution — a system that solves several problems at once is needed. The study is published in the journal Burns and trauma.
What did you come up with?
The researchers created a DPFI hydrogel, essentially a “smart bandage” that can be injected with a syringe. Inside is the natural flavonoid dihydromyricetin (DMY), packed into PF127-CHO polymer micelles, and the gel itself is cross-linked with PEI polymer. This composition provides three key properties:
- Antibacterial action from the first minutes (due to PEI).
- Long-term tissue “feeding” of DMY: it neutralizes ROS, shifts macrophages from the inflammatory profile M1 to the “repair” M2, reduces levels of pro-inflammatory cytokines and supports vascular growth and epithelialization.
- Bonus for glucose metabolism: DMY is known as a compound that helps control hyperglycemia - in the model it improved the "sugar background" of healing.
How does this work in practice?
Gel:
- Sticks to damp fabric, self-heals after deformation, passes through a needle (shear liquefaction) and slowly releases DMY.
- In cellular tests, it suppressed the growth of MRSA and E. coli, sharply reduced ROS, “quenched” inflammation (down IL-6/IL-1β/TNF-α, up IL-10/IL-4), accelerated the migration of fibroblasts and the formation of “tubes” by the endothelium (vascular rudiments).
- In experiments on diabetic mice with MRSA-infected wounds, it accelerated closure: by the 15th day, one of the formulas gave ~97% healing (versus ~65% without treatment), enhanced collagen formation and microvascular growth, and reduced blood glucose levels during healing.
How is this bandage different from the usual one?
Conventional hydrogels are basically a moist environment and a barrier. Here is a programmable platform: first a blow to microbes, then an antioxidant and anti-inflammatory block, and then support for the growth of blood vessels and epithelium. Plus, an effect on glycemia, which is important specifically for diabetic wounds.
Where are the pitfalls?
Everything is shown in vitro and on mice. Before the clinic you need to:
- confirm safety (PEI has toxicity limitations - dosage and form are important),
- to test the bioavailability and stability of DMY in real dressings,
- conduct large animal trials and then clinical studies in foot ulcers.
Authors' comments
On the novelty of the platform.
“To our knowledge, DPFI is the first hydrogel that purposefully combines DMY + PEI + PF127-CHO into a multifunctional framework with programmable action.”Why “programmability”?
The authors emphasize the sequence of effects: rapid bacterial suppression (PEI) → ROS clearance and inflammation relief (DMY, M1→M2 switching) → stimulation of angiogenesis/epithelialization while modulating glycemia.
“DPFI provides a sequential therapy targeting key nodes of diabetic wound healing.”Multi-target action confirmed.
“Comprehensive in vitro and in vivo testing demonstrated DPFI’s antibacterial, antioxidant and anti-inflammatory properties, as well as support for cell proliferation, vascularization and glucose regulation.”On clinical potential:
“DPFI is a promising integrative strategy for improved management of chronic diabetic wounds and deserves further exploration for clinical application.”About “dose as a tool”.
According to the authors, a higher DMY content suppresses inflammation and bacterial load more strongly, while a lower one better supports angiogenesis. Load optimization for the healing phase is needed.On safety.
The authors note good biocompatibility (hemolysis <5% by ISO), no noticeable toxicity in animal organs, and the adhesive and self-healing properties of the gel, which are convenient for dressings.Limitations and next step:
“Results are in cells and mice; large animal studies, pharmacokinetic/stability studies, fine-tuning of formulation (including potential toxicity of PEI), and then early clinical trials (e.g. in foot ulcers) are needed.”
Summary
DPFI is a promising “multi-tool” dressing for complex diabetic wounds: it simultaneously fights bacteria, relieves oxidative and inflammatory stress, supports vascular growth, and helps keep sugar under control. It’s still a long way from the first aid kit, but the concept seems logical for a problem where one measure is almost never enough.
