
All iLive content is medically reviewed or fact checked to ensure as much factual accuracy as possible.
We have strict sourcing guidelines and only link to reputable media sites, academic research institutions and, whenever possible, medically peer reviewed studies. Note that the numbers in parentheses ([1], [2], etc.) are clickable links to these studies.
If you feel that any of our content is inaccurate, out-of-date, or otherwise questionable, please select it and press Ctrl + Enter.
Scientists discover key protein responsible for brain asymmetry
Last reviewed: 02.07.2025
 ">
">The genetic mechanisms underlying unique left-right differences in the brain are now better understood thanks to new research, paving the way for a better understanding of human disorders associated with abnormal brain asymmetry.
A protein called Cachd1 plays a key role in establishing the different neural structure and function on each side of the brain, researchers from UCL, the Wellcome Sanger Institute, the University of Oxford and other co-authors have found. The study is published in the journal Science.
By conducting genetic experiments on zebrafish, the researchers found that when Cachd1 is mutated, the right side of the brain loses its normal asymmetric development and becomes a mirror image of the left side. This disorder causes abnormal neural connectivity, which affects brain function.
This discovery sheds light on the genetic mechanisms underlying brain asymmetry, a phenomenon observed in many animal species, including humans. Understanding these processes may lead to a better understanding of human disorders in which brain asymmetry is disrupted, such as schizophrenia, Alzheimer's disease, and autism spectrum disorders.
Despite their mirror-image anatomy, the left and right hemispheres of the human brain have functional differences that affect neural connections and cognitive processes such as language. How these left-right differences in neural circuitry arise is still poorly understood.
Using zebrafish—a well-known model organism for studying brain development due to their transparent embryos—the researchers set out to study how Cachd1 might influence brain asymmetry.
The team found that when Cachd1 is mutated, a region of the brain called the habenula loses its normal left-right distinction. Neurons on the right side become similar to neurons on the left side, which disrupts neural connections in the habenula and potentially affects its function.
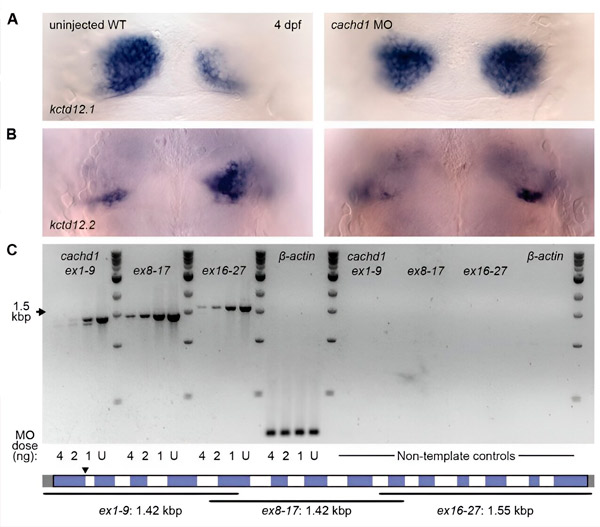
Knockdown of cachd1 using morpholinos results in bilateral symmetry. (AB) Dorsal view at 4 days post-fertilization in uninjected wild type and cachd1 morpholino-injected larvae after whole-mount in situ hybridization using antisense riboprobes against the asymmetric dorsal habenula marker kctd12.1. (C) Semi-quantitative RT-PCR of cachd1 transcripts. Source: Science (2024). DOI: 10.1126/science.ade6970
Protein binding experiments showed that Cachd1 binds to two receptors that allow cells to communicate through the Wnt signaling pathway, one of the most intensively studied cellular communication pathways, which plays important roles in early development, stem cell formation, and many diseases.
Furthermore, the effects of Cachd1 appear to be specific to the right side of the brain, suggesting that there is an unknown inhibitory factor that limits its activity on the left side. Although the full details are not yet clear, the data strongly suggest that Cachd1 plays a key role in establishing the distinction between the left and right sides of the developing brain by regulating cellular communication specifically on the right side.
Future studies will examine whether Cachd1 has other important functions related to the Wnt pathway.
“This was a highly collaborative project that benefited greatly from an interdisciplinary approach – genetics, biochemistry and structural biology came together to better understand the establishment of left-right asymmetry in the brain, as well as identifying a new component of an important signalling pathway with multiple roles in health and disease,” says Dr Gareth Powell, co-author of the study, a former Wellcome Sanger Institute PhD student and now a member of UCL’s Department of Cell and Developmental Biology.
“I am delighted to see the publication of this highly collaborative study, which brought together many talented people with different research interests and skills from different institutes. Together, the team has enabled us to make exciting new discoveries about both the Wnt signalling pathway and the development of brain asymmetry,” said Professor Steve Wilson, senior author of the study and a member of UCL’s Department of Cell and Developmental Biology.
