
All iLive content is medically reviewed or fact checked to ensure as much factual accuracy as possible.
We have strict sourcing guidelines and only link to reputable media sites, academic research institutions and, whenever possible, medically peer reviewed studies. Note that the numbers in parentheses ([1], [2], etc.) are clickable links to these studies.
If you feel that any of our content is inaccurate, out-of-date, or otherwise questionable, please select it and press Ctrl + Enter.
Immune response triggered by plant virus effectively destroys cancer cells
Last reviewed: 27.07.2025
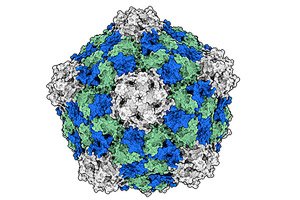 ">
">A virus that commonly infects black-eyed peas is showing huge potential as a cheap, powerful immunotherapy against cancer — and scientists reveal why.
In a study published in Cell Biomaterials, a team led by chemical and nanoengineering experts at the University of California, San Diego, took a closer look at why cowpea mosaic virus (CPMV) — unlike other plant viruses — is uniquely effective at activating the immune system to recognize and destroy cancer cells.
The study is titled: "Comparative Analysis of Plant Viruses for the Development of Anticancer Immunotherapeutic Drugs" and is published in the journal Cells Biomaterials.
Antitumor effect of CPMV
In preclinical studies, CPMV has demonstrated potent antitumor activity in various mouse models as well as in dogs with cancer. When administered directly into tumors, CPMV recruits innate immune cells — such as neutrophils, macrophages, and natural killer cells — into the tumor microenvironment to destroy tumor cells.
This activates B cells and T cells, creating systemic and long-term immune memory. This “reboot” of the immune system not only helps destroy the target tumor, but also primes the body to seek out and eliminate metastases in other parts of the body.
“It’s striking that it’s CPMV, and not other plant viruses, that triggers an antitumor response,” says Nicole Steinmetz, who holds the Leo and Trude Szilard Chair in the Jacobs School of Engineering and the Department of Chemical and Nanoengineering at UC San Diego and lead author of the study.
"This study gives us insight into why CPMV works so effectively," added first author Anthony Omole, a graduate student in Steinmetz's lab.
“The most exciting thing was that even though CPMV does not infect human immune cells, they still respond to it and become reprogrammed into an active state, which ultimately trains them to identify and destroy cancer cells.”
What is the secret of CPMV?
The key question in translating CPMV into human cancer treatment is: What makes this plant virus so effective in fighting cancer?
To find out, Omole, Steinmetz, and their colleagues at the National Cancer Institute's (NCI) National Nanotechnology Characterization Laboratory compared CPMV with cowpea chlorotic spot mosaic virus (CCMV), a closely related plant virus that has no antitumor activity when injected into tumors.
Both viruses have similar-sized particles and are taken up by human immune cells at the same rate. However, inside the cell, the reactions are different.
How does CPMV work differently?
CPMV stimulates interferons types I, II and III, proteins with well-known anti-cancer properties.
“This is particularly interesting because the first cancer immunotherapy drugs were recombinant interferons,” Omole noted.
CCMV, on the other hand, activates proinflammatory interleukins, which do not lead to effective tumor killing.
Viruses are also processed differently inside mammalian cells:
- CPMV RNA persists longer and enters the endolysosome, where it activates toll-like receptor 7 (TLR7), a key element in triggering the antiviral and antitumor immune response;
- CCMV RNA does not reach this activation point and, accordingly, does not trigger the necessary immune mechanisms.
Advantage in production
An additional advantage of CPMV is that it can be a low-cost immunotherapeutic. Unlike many current drugs that require complex and expensive production, CPMV can be grown using molecular farming.
"It can be grown in plants using only sunlight, soil and water," Omole said.
Next steps: clinical trials
The team is working to move CPMV into clinical trials.
"This study provides important insight into the mechanism of action of CPMV. We are now actively preparing for the next steps to select the most effective candidate that provides both antitumor effect and safety," Steinmetz said.
"Now is the time. We are ready to move from laboratory research to clinical trials."
