
All iLive content is medically reviewed or fact checked to ensure as much factual accuracy as possible.
We have strict sourcing guidelines and only link to reputable media sites, academic research institutions and, whenever possible, medically peer reviewed studies. Note that the numbers in parentheses ([1], [2], etc.) are clickable links to these studies.
If you feel that any of our content is inaccurate, out-of-date, or otherwise questionable, please select it and press Ctrl + Enter.
How an embryo 'bites' into maternal tissue: the mechanics of implantation in humans filmed in real time for the first time
Last reviewed: 23.08.2025
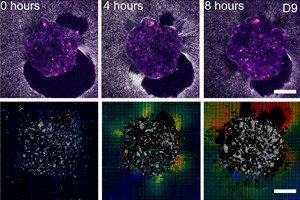 ">
">Scientists from Barcelona (IBEC, Dexeus Mujer) and Tel Aviv have shown for the first time in real time and in 3D how a human embryo attaches to the "uterine scaffold" and literally pulls and restructures the surrounding tissue. To do this, they created a deformable ex vivo platform (collagen/ECM gels) and applied traction force microscopy directly to living human and mouse embryos. The key finding: the pattern of forces is species-specific, and the embryos themselves are mechanosensitive: they respond to external mechanical cues by restructuring the cytoskeleton and changing the orientation of growth.
Background of the study
Implantation is the "bottleneck" of human reproduction: it is at this stage that both natural conceptions and IVF attempts most often fail. At the same time, human implantation is interstitial: the embryo does not simply "stick" but is completely embedded in the endometrium - a biochemically and mechanically complex process, but until recently almost not observed in living systems in humans. Therefore, the mechanics of adhesion and invasion remained a "black box", and conclusions were often made based on indirect markers or data from animal models.
Classical implantation biology has relied heavily on the mouse, but there are fundamental differences between species, from blastocyst orientation to depth of implantation and pattern of cellular forces. In mice, implantation is more “superficial,” with preferential directions of tissue displacement; in humans, it is distinctly invasive, with multifocal traction forces around the embryo. These differences suggest that the mouse model does not always scale to humans, especially when it comes to mechanics. Direct observations of the human embryo in a deformable environment were needed.
The technological breakthrough was made possible by combining deformable 2D/3D matrices (collagen/ECM) and traction force microscopy with long-term high-frequency imaging. This “artificial womb” made it possible to literally see and measure how the embryo pulls, restructures and “bores” the surrounding tissue – and how it responds to external mechanical cues (mechanosensitivity). This opens the way to new criteria for assessing implantation potential and to fine-tuning the conditions of embryo transfer.
The context is applied: if the mechanical properties of the environment and the pattern of embryonic forces are associated with the success of implantation, then in IVF it is possible to purposefully select the rigidity/composition of the matrix, take into account the time windows of transfer, and even use “force” metrics as an additional selection marker. In parallel, such platforms will help to explain the proportion of early pregnancy losses, when the biochemistry is “normal”, but the mechanics of adhesion are not. All this makes direct 3D observations of human implantation not just a beautiful video, but a new tool for reproductive medicine.
Why is this important?
Implantation failure is one of the main causes of infertility and up to 60% of spontaneous miscarriages. Despite biochemical progress in IVF, the mechanics of this process in humans remained a "black box". A new approach allows us to see the forces and trajectories of embryo implantation and provides a basis for improving embryo selection and transfer conditions.
How it was done
The researchers assembled an "artificial womb" - a soft, transparent and deformable environment in which a tissue-like matrix visibly shifts under the influence of embryonic forces. Next came continuous microscopy and computational analysis of the fibre displacements.
- 2D and 3D platforms: in 3D, the embryo is immediately embedded into the matrix (the attachment stage is “bypassed”), which allows one to see the drilling into the thickness of the tissue.
- High "survival and penetration" in 3D: about 80% successful invasion (limited by proximity to glass).
- Traction maps and digital volume correlation show the amplitudes and directions of displacements around the embryo - essentially a "print" of force over time.
What exactly was found (briefly and point by point)
1) Species-specific mechanics of implantation
- Human: the embryo is inserted into the matrix, creating multiple foci of traction and forming radially uniform displacements around itself; the depth of invasion is up to 200 µm.
- Mouse: the embryo mainly spreads over the surface with pronounced principal displacement directions.
2) The embryo senses the mechanics of the environment
- External forces → answer: in the human embryo - recruitment of myosin and directed cell pseudopodia; in the mouse - rotation of the implantation/growth axis towards the source of external force (orientation of the PD axis).
- Mechanosensitive markers: in mouse, shifts in YAP localization in trophoblast; together this indicates a mechanosensitive feedback circuit.
3) The relationship between strength and success of implantation
- Less collagen displacement → worse implantation progress in human embryos.
- Integrins - the "coupler" of strength: RGD peptide blockade/Src inhibition in mice reduces implantation depth/area.
What does implementation look like?
- On 2D and 3D platforms, a growing “halo” of fiber displacements forms around the embryo; the traction map pulsates as if the embryo is “scanning” its surroundings.
- On glass, the human embryo forms a flat outgrowth, but in a soft matrix it remains more spherical and goes deeper - as in living tissue.
What does this give to practice (prospects for IVF and not only)
The idea is simple: implantation is not only "receptor chemistry", but also the mechanics of adhesion and traction. This means that we can optimize:
- Materials and medium hardness during culture/implantation potential tests;
- New markers for embryo selection - based on trajectories and amplitude of displacements in the “smart” matrix;
- Uterine training/modulation (e.g. through gentle mechanical cues) to improve adhesion without aggressive interventions.
Caution: ex vivo work is not "inside the womb". But the very fact that an external mechanical signal changes the orientation of implantation/organization of the axes opens the way to personalized conditions of embryo transfer.
Restrictions
- The ex vivo model does not take into account the immune, hormonal and vascular dynamics of the real endometrium;
- Matrigel/collagen define a set of properties (rigidity, viscoelasticity, composition), it is difficult to change them by one parameter;
- Ethical constraints for human studies (up to 14 days window) limit long-term observation. However, the high agreement with known in vivo implantation modes (interstitial in humans vs. superficial in mice) increases confidence in the model.
Conclusion
The human embryo actively "pulls" and "bores" its way into the maternal tissue, and mechanical cues from the environment can reconfigure its behavior. The pattern of forces and the strategy of implantation are different in humans and mice - and this may explain why the mouse model does not always predict successful implantation in humans. Mechanics is now a full-fledged player in early embryology and reproductive medicine.
Source: Godeau AL et al. Traction force and mechanosensitivity mediate species-specific implantation patterns in human and mouse embryos. Science Advances 11(33): eadr5199 (15 August 2025). DOI: 10.1126/sciadv.adr519
