
All iLive content is medically reviewed or fact checked to ensure as much factual accuracy as possible.
We have strict sourcing guidelines and only link to reputable media sites, academic research institutions and, whenever possible, medically peer reviewed studies. Note that the numbers in parentheses ([1], [2], etc.) are clickable links to these studies.
If you feel that any of our content is inaccurate, out-of-date, or otherwise questionable, please select it and press Ctrl + Enter.
Genetic and metabolic aspects of the pathogenesis of osteoarthritis
Medical expert of the article
Last reviewed: 08.07.2025
 ">
">The role of mechanical factors in the pathogenesis of osteoarthritis is undeniable, but there is convincing evidence that some forms of osteoarthritis are inherited according to Mendel's laws. Hereditary osteoarthropathies can be divided into:
- primary generalized osteoarthritis (PGAO),
- crystal associated arthropathies,
- premature osteoarthritis due to hereditary osteochondrodysplasia.
In 1803 W. Heberden described "slightly dense nodes, the size of a small pea" on the dorsal surface of the distal interphalangeal joints of the hands. This symptom, according to the author, distinguishes osteoarthritis from other joint diseases, including gout. J. Hayagarth (1805) expanded the clinical description of Heberden's nodes, noting their frequent association with arthrosis of other localizations. Later Bouchard described similar nodes on the dorsal surface of the proximal interphalangeal joints of the hands. Using the term "Heberden's and Bouchard's nodes", W. Osier distinguished "hypertrophic arthritis" and "deforming arthritis" (1909). In 1953 RM Stecher and H. Hersh discovered the prevalence of Heberden's nodes among family members and concluded that they are inherited in an autosomal dominant manner. Subsequent studies following the discovery by RM Stecher and H. Hersh revealed an association of Heberden's and Bouchard's nodes with degenerative lesions of other joints. Based on clinical examination data and HLA typing, JS Lawrence (1977), JS Lawrence et al. (1983) suggested the presence of polygenic inheritance rather than a single gene defect.
The phenotypic spectrum of hereditary osteoarthritis varies widely from mild forms that become clinically apparent only in late adulthood to very severe forms that manifest in childhood. Traditionally, all of these forms have been classified as secondary osteoarthritis. It is now known that some of these phenotypes are caused by mutations in genes encoding macromolecules of the articular cartilage ECM, which disrupt the integrity of the cartilage matrix and the regulation of chondrocyte proliferation and gene expression. These hereditary diseases represent a distinct subgroup of osteoarthritis that is distinct from secondary osteoarthritis.
Differences between hereditary and secondary osteoarthritis (according to Williams CJ and Jimenez SA, 1999)
Hereditary osteoarthritis |
Secondary osteoarthritis |
|
Etiology |
Mutation of genes expressed in articular cartilage |
Various hereditary and acquired diseases |
Pathogenesis |
Damage to the structural or functional components of articular cartilage |
Secondary manifestations of the disease, which does not always affect only articular cartilage |
Treatment |
Gene therapy may be possible to correct the gene defect |
Treatment of the underlying disease |
Chondrodysplasia/osteochondrodysplasia is a group of clinically heterogeneous diseases characterized by abnormalities in the growth and development of articular cartilage and growth plate. Some CD/OCD lead to early development of osteoarthritis, clinically characterized by a severe course. Among them, the following diseases can be distinguished:
- spondyloepiphyseal dysplasia (SED),
- Stickler syndrome,
- dysplasia Knista,
- multiple epiphyseal dysplasia (MED),
- metaphyseal chondrodysplasia (MCD),
- some oto-spondylo-meta-epiphyseal dysplasias (OSMED).
Hereditary dysplasias characterized by early onset osteoarthritis (according to Williams CJ and Jimenez SA, 1999)
Disease |
Locus |
Type of inheritance |
Mutated gene |
Type of mutation |
Early OA with late onset SED (OAR)* |
12q13.1-q13.2 |
HELL |
COL 2 A, |
Base substitution, insertion, deletion |
Stickler syndrome (STL1) |
12q13.1-q13.2 |
HELL |
COL2A1 |
Replacement of the base, insertion |
Stickler syndrome (STL2) |
6р21.3 |
HELL |
COLA |
Insertion, deletion |
Stickler syndrome |
1p21 |
HELL |
COLA |
Replacement of the base |
Wagner syndrome |
12q13.1-q13.2 |
HELL |
COUA, |
Replacement of the base |
OSMED |
6р21.3 |
AR |
COLA |
Replacement of the base |
Marshall syndrome |
1p21 |
HELL |
COLA |
Insert |
Knista dysplasia |
12q13.1-q13.2 |
HELL |
COLA |
Insertion, deletion |
M3fl(EDM1) |
19р13.1 |
HELL |
COMP |
Replacement of the base |
MED (EDM 2) |
1р32.2-рЗЗ |
HELL |
COLA |
Insert |
MCDS |
6q21-q22.3 |
HELL |
COLA |
Base substitution, deletion |
MCDJ Jansen |
Зр21.2-р21.3 |
HELL |
PTHR, |
Replacement of the base |
*Locus symbols are given in brackets; AD - autosomal dominant; AR - autosomal recessive.
Spondyloepiphyseal dysplasia
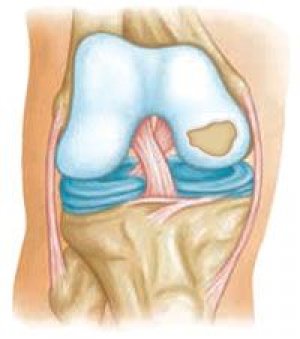
Spondyloepiphyseal dysplasias (SED) include a heterogeneous group of diseases with an autosomal dominant type of inheritance, characterized by abnormal development of the axial skeleton and severe changes in the epiphyses of long tubular bones, often causing dwarfism. SED often has a severe clinical course, accompanied by shortening of the body and, to a lesser extent, limbs.
In forms of EDS that manifest at a later age, the phenotype is often little changed and may not manifest clinically until adolescence, when severe osteoarthrosis develops. Deformity of the lumbar spine may manifest as narrowing of the intervertebral discs, platyspondyly, and minor kyphoscoliosis. Anomalies of the epiphyses in the peripheral joints and early degenerative changes in them are also detected. The most constant sign of peripheral joint damage is flattening of the articular surfaces of the ankle and knee joints, as well as flattening of the intercondylar groove of the femur. Anomalies of the head and neck of the femur are often detected with the development of osteoarthrosis of the hip joint, manifesting in adolescence.
Since type II collagen is the major component of the hyaline cartilage ECM, it has been suggested that the gene encoding it, COL1A, is the cause of EDS. The first description of a genetic link between the phenotype of early osteoarthritis associated with late-onset EDS and the procollagen type II gene, COL 2 A, dates back to 1989 and 1990. The first report of a COL 2 A mutation in relatives with early osteoarthritis associated with late-onset EDS involved the Arg519>Cys base substitution. To date, four more families with similar mutations have been identified. In members of another family with early OA and mild EDS, the Arg75>Cys base substitution was found, although the EDS phenotype in members of this family is not similar to the phenotype of the family with an arginine to cysteine substitution at position 519. Other mutations COL 2 A-Gly976>Ser, Gly493>Ser were also found in members of families with EDS. J. Spranger et al. (1994) used the term "type 11 collagenopathy" to describe hereditary diseases of cartilage tissue with a primary mutation in the procollagen type II gene COL1A.
Classic form of Stickler syndrome
It was first described in 1965 by G. B. Stickler and colleagues, who called it hereditary arthro-ophthalmopathy. The syndrome described by G. B. Stickler was characterized by visual impairment and severe degenerative joint disease, which usually develops in the third or fourth decade of life. It is an autosomal dominant disorder with an incidence of approximately 1 in 10,000 live births. The clinical presentation includes myopia, progressive deafness, cleft palate, hypoplasia of the mandible (Pierre-Robin anomaly), and hypoplasia of the epiphyses. In the neonatal period, radiographs of patients with Stickler syndrome reveal enlarged epiphyses, primarily the proximal femur and distal tibia. During growth, epiphyseal dysplasia develops, which is manifested by irregular ossification of the epiphyses and subsequent degenerative changes.
Since COL 2 A is expressed in articular cartilage and the vitreous body of the eyeball, the occurrence of Stickler syndrome was associated with the pathology of this gene. However, an examination of several families with Stickler syndrome showed that not all families have a disease associated with COL 2 A. This form of the disease is called type I Stickler syndrome (locus symbol STL1).
The spectrum of clinical manifestations of Stickler syndrome varies widely, and several phenotypes have been identified to date. Among them is Wagner syndrome, which is characterized by a predominance of damage to the eyeball; OA in Wagner syndrome virtually never develops, although a mutation of the COL 2 A gene (base substitution Gly67>Asp) has been identified in patients. It remains unclear why such a COL mutation compromises only the function of the vitreous body and does not affect the hyaline cartilage.
Another form of Stickler syndrome is the so-called Dutch variant; it is characterized by all the classic manifestations of the syndrome except for the visual impairment. HG Brunner et al. (1994) showed that the Dutch phenotype of Stickler syndrome is associated with a mutation in the COL,,A 2 gene: the dominant mutation is a 54-base pair deletion followed by an exon deletion. M. Sirko-Osadsa et al. (1998) reported another family, unrelated to the one described by the previous authors, with a similar phenotype and a mutation in the COL,,A 2 gene (27-base pair deletion), which confirms the data of HG Brunner et al. (1994). This variant is called type II Stickler syndrome (locus symbol STL1).
Recently, a third locus of Stickler syndrome was identified in members of a family with vitreous and retinal pathology that are phenotypically significantly different from the changes observed in the "classic" variant of the syndrome. A mutation in the COL2A| gene (base substitution Gly97>Val) was found in members of this family. Of course, new descriptions of cases of this pheno- and genotype of Stickler syndrome are needed to confirm the findings of AJ Richards et al.
The nosological connection between Marshall syndrome and the classic version of Stickler syndrome has been discussed for a long time. Now Marshall syndrome is classified as a separate phenotype mainly due to the more pronounced deformation of the facial skeleton, although the damage to the peripheral joints is similar to that in type I Stickler syndrome. In Marshall syndrome, osteoarthritis of the knee joints and lumbosacral spine begins after 30 years. The cause of the syndrome is a mutation in the type IX collagen gene COL n A1.
 [ 1 ], [ 2 ], [ 3 ], [ 4 ], [ 5 ], [ 6 ]
[ 1 ], [ 2 ], [ 3 ], [ 4 ], [ 5 ], [ 6 ]
OSMED
This phenotype was described in a Dutch family in which degenerative changes in the joints resembling osteoarthrosis appeared in adolescence and affected mainly the hip, knee, elbow and shoulder joints; peculiar facial features, increased lumbar lordosis, enlarged interphalangeal joints, and hearing loss were also found, but no visual anomalies were detected (Vikkula M. et al., 1995). The researchers found a mutation in the gene encoding the a 2 -chain of type II collagen COL,,A 2.
Knista dysplasia
Characterized by shortening of the trunk and limbs, flattening of the face and bridge of the nose, exophthalmos, and severe joint abnormalities. In patients with Kniest syndrome, the joints, usually large from birth, continue to enlarge in childhood and early adolescence. They also often have myopia, hearing loss, cleft palate, and clubfoot; most patients develop severe degenerative changes early, especially pronounced in the knee and hip joints. Spinal radiographs reveal flattening and significant elongation of the vertebral bodies and platyspondyly. The long tubular bones are deformed like a dumbbell, and ossification of the epiphyses is slow. In the joints of the hands, the epiphyses are flattened and the joint spaces are narrowed. The articular cartilage is soft, its elasticity is reduced; histologically, large cysts are found in it (the "Swiss cheese" symptom). Kniest syndrome is caused by a mutation in the procollagen type II gene COb2A1.
 [ 7 ], [ 8 ], [ 9 ], [ 10 ], [ 11 ], [ 12 ], [ 13 ], [ 14 ]
[ 7 ], [ 8 ], [ 9 ], [ 10 ], [ 11 ], [ 12 ], [ 13 ], [ 14 ]
Multiple epiphyseal dysplasia (MED)
A heterogeneous group of diseases characterized by abnormal development of the growth plates of long tubular bones, as well as early (manifesting in childhood) severe osteoarthrosis affecting both axial and peripheral joints (most often knee, hip, shoulder and hand joints). Clinically, MED manifests itself as pain and stiffness in the joints, changes in gait. Patients with MED also have minimal changes in the spinal column (various degrees of flattening of the vertebral bodies), sometimes the spine is intact. Short stature of patients is also characteristic, although dwarfism rarely develops. The visual organ is not affected. MED include several variants, for example, the Fairbanks and Ribbing phenotype.
MEDs are inherited in an autosomal dominant manner with varying degrees of penetrance. Since the hallmark of MEDs is an anomaly of the epiphyseal growth plate, it has been suggested that these dysplasias are caused by a defect in the genes encoding macromolecules of the growth plate cartilage. It turned out that at least three loci are associated with the MED phenotype. Studies by E.J. Weaver et al. (1993), J.T. Hecht et al. (1992) excluded the genes of collagen types II and VI, the core protein of proteoglycans, and the connective protein of cartilage from the list of "culprits" of MEDs. JT Hecht et al. (1993), R. Oehelmann et al. (1994) found a link between MED, as well as the clinically related pseudoachondroplasia syndrome, and the pericentromeric region of chromosome 19. Subsequent studies identified a mutation in the gene encoding cartilage oligomeric matrix protein (OMMP) in three patients with MED (locus symbol EDM1). Since all three mutations occurred in the gene region encoding the calcium-binding domain of OMMP, it is likely that the calcium-binding function of this protein is essential for normal development of growth plate cartilage.
M. D. Briggs et al. (1994) reported a Dutch family with an MED phenotype associated with a region of chromosome 1 containing one of the type IX collagen genes, COL1A1 (symbol of the EDM 2 locus). Notably, the mutation found was the first evidence of a role for type IX collagen, localized on the surface of collagen II fibrils, in maintaining the integrity of hyaline cartilage. M. Deere et al. (1995) showed that the Fairbanks phenotype was not genetically associated with either the EDM or EDM2 locus, confirming the heterogeneity of MED.
Metaphyseal chondrodysplasia (MCD)
A heterogeneous (more than 150 types have been described) group of hereditary diseases of the hyaline cartilage, which clinically manifest as early osteoarthrosis. MHDs are characterized by changes in the bone metaphyses. Clinically, they manifest as short stature, shortened limbs, bowed shins, and a "duck" gait. Patients with MHD also show signs of damage to other systems (for example, the immune and digestive systems). Disorganization of the growth plate cartilage is observed, which histologically manifests itself as clusters of proliferated and hypertrophied chondrocytes surrounded by thickened septa and disorganized matrix, as well as penetration of non-calcified cartilage into the subchondral bone.
Jansen, Schmid and McKusick syndromes are the most well-studied MHDs. They are similar in the features of skeletal anomalies, but differ in severity (Jansen syndrome-McKusick syndrome-Schmid syndrome). The most common is Schmid syndrome (symbol of the MCDS locus), which is inherited in an autosomal dominant manner. Radiologically, the syndrome is manifested by coxa vara, shortening and curvature of the tubular bones, cup-shaped deformation of the metaphyses (more pronounced in the proximal than in the distal part of the femur). The most pronounced changes are observed in the growth plates of the long tubular bones.
At least 17 different types of collagen X gene mutations have been described in patients with Schmid syndrome. Collagen X is expressed in hypertrophied chondrocytes of the growth plates and may be involved in ossification processes. Thus, a mutation in the collagen X gene COb2A1 is the most likely cause of Schmid syndrome.
Children with Jansen syndrome have hypercalcemia, elevated urinary phosphate levels, and decreased parathyroid hormone (PTH) and PT-related peptide levels. The anomaly of the latter is probably responsible for the development of Jansen syndrome. In 1994, A. S. Karaplis and co-authors published the results of an original study. After disruption of the gene encoding PT-related peptide in mouse embryonic stem cells, mice with a deficiency in this allele died immediately after birth. They were found to have an anomaly in subchondral bone development, impaired cartilage growth, and decreased chondrocyte proliferation. In 1995, E. Schipani and co-authors reported a heterozygous mutation in the PTH receptor gene in a patient with Jansen syndrome. The mutation consisted of a Gys223>Arg base substitution, which led to cAMP accumulation; This means that the amino acid histidine at position 223 plays a crucial role in signal transmission. Later, E. Schipani et al. (1996) reported three other patients with Jansen syndrome, two of whom had a similar mutation, and the third had a TrА10>Рrо substitution.
Primary generalized osteoarthritis
The most common hereditary form of osteoarthritis is primary generalized osteoarthritis (PGOA), which was first described as a separate nosology by JH Kellgren and R. Moore in 1952. Clinically, primary generalized osteoarthritis is characterized by the appearance of Bouchard's and Heberden's nodes, polyarticular lesions. Primary generalized osteoarthritis is characterized by the early onset of osteoarthritis manifestation and its rapid progression. Radiologically, primary generalized osteoarthritis does not differ from non-hereditary osteoarthritis. Despite the fact that the issue of the etiopathogenesis of primary generalized osteoarthritis is still debated, the studies demonstrate the important role of hereditary predisposition in the occurrence and progression of primary generalized osteoarthritis.
Thus, JH Kellgren et al. (1963) found Boucharay-Heberden nodes in 36% of male relatives and 49% of female relatives, while in the general population these figures were 17 and 26%, respectively. In individuals with primary generalized osteoarthritis, the HLA A1B8 haplotype and the MZ isoform of a1-antitrypsin are more often detected. In a classic study involving twins, TD Spector et al. (1996) performed radiography of the knee joints and hand joints in 130 monozygotic and 120 fraternal female twins for changes characteristic of osteoarthritis. It turned out that the concordance of radiographic signs of osteoarthritis of all localizations was 2 times higher in monozygotic twins compared to fraternal twins, and the contribution of genetic factors ranged from 40 to 70%. A study of nodular osteoarthritis by GD Wright et al. (1997) demonstrated early onset of the disease, high severity, and a negative correlation between the age of onset of the disease in patients and the age of conception of their parents.
Among crystal-associated arthropathies, the deposition of uric acid crystals and calcium-containing crystals in the joint cavity have a familial predisposition.
Hereditary crystal-associated arthropathies (according to Williams C.J. and Jimenez S.A., 1999)
Disease |
Locus |
Type of inheritance |
Mutated gene |
Type of mutation |
Gout (HPRT)* |
Xq27 |
X-linked |
HPRT1 |
Base substitution, deletion |
Gout (PRPS) |
Xq22-q24 |
X-linked |
PRPS1 |
Replacement of the base |
Primary pyrophosphate arthropathy (CCAL1) |
5р15.1-р15.2 |
HELL |
? |
? |
Early-onset pyrophosphate arthropathy associated with 0A (CCAL2) |
8q |
HELL |
? |
? |
*Locus symbols are given in brackets; AD – autosomal dominant.
In 1958, D. Zintann S. Sitaj presented clinical descriptions of a pathology they called "chondrocalcinosis" in 27 patients. Most of the patients belonged to five families, indicating a hereditary component in the etiopathogenesis of the disease. Later, D. McCarty and J. L. Hollander (1961) reported on two patients who were suspected of having gout with deposition of nonurate crystals in the joint cavity. X-ray examination revealed abnormal calcification of the hyaline cartilage of many joints.
Radiographically, calcium pyrophosphate dihydrate crystal deposition disease, or pyrophosphate arthropathy, resembles sporadic OA, but it more often affects joints that are not typical for common forms of osteoarthrosis (e.g., metacarpophalangeal, scaphoradial, patellofemoral knee joints). In pyrophosphate arthropathy, subchondral bone cysts are more often formed. Although in most cases, chondrocalcinosis occurs before the manifestation of secondary osteoarthrosis, in some individuals the disease may begin as idiopathic osteoarthrosis, which is accompanied by metabolic disorders (hemochromatosis, hyperparathyroidism, hypomagnesemia, etc.).
Most likely, structural changes in the ECM of articular cartilage induce the deposition of calcium pyrophosphate dihydrate crystals. A. O. Bjelle (1972, 1981) found a decrease in collagen content and fragmentation of collagen fibers in the middle zone of the matrix of articular cartilage of Swedish family members with pyrophosphate arthropathy. Since these areas did not contain crystals, the authors suggested that the described matrix anomaly may predispose to their deposition and the development of degenerative changes in the joints. Based on a study of sporadic cases of pyrophosphate arthropathy, K. Ishikawa et al. (1989), I. Masuda et al. (1991) concluded that chondrocalcinosis is caused by a mutation in the genes encoding ECM proteins. CJWilliams et al. (1993), AJ Reginato et al. (1994) found a heterozygous mutation COL 2 A, (base substitution Argl5>Cys) in members of a large family with a clinical phenotype of severe early osteoarthritis with ankylosis, late development of spondyloepiphyseal dysplasia and chondrocalcinosis of hyaline and fibrocartilage. However, it turned out that in members of this family chondrocalcinosis was secondary to OA.
It has also been suggested that inorganic components of the ECM contribute to crystal formation. For example, hypomagnesemia causes chondrocalcinosis by inhibiting the enzyme pyrophosphatase, which in turn reduces crystal dissolution. Elevated levels of inorganic phosphates have been found in the synovial fluid of patients with pyrophosphate arthropathy. This and other observations have suggested that patients with pyrophosphate arthropathy have a local disorder of pyrophosphate metabolism. The enzyme nucleoside triphosphate pyrophosphohydrolase has been described, which may be involved in the formation of pyrophosphate crystals in the area of their deposition in the ECM. Elevated levels of this enzyme have been found in sporadic cases of pyrophosphate arthropathy, but this abnormality has not been observed in familial forms of the disease (Ryan LM et al., 1986). However, when culturing fibroblasts and lymphoblasts from patients with familial pyrophosphate arthropathy, an increase in the content of inorganic phosphates was detected, which also confirms the assumption about the role of disturbances in local pyrophosphate metabolism in the pathogenesis of the disease.
In recent years, attempts have been made to identify genes "guilty" of the occurrence of familial cases of pyrophosphate arthropathy. Thus, analysis of genetic material obtained from members of a large family with pyrophosphate arthropathy (Maine, USA), in which chondrocalcinosis developed secondary to severe, rapidly progressing, non-dysplastic osteoarthrosis, excluded a connection between the disease and the COL 2 locus. However, the authors of this study found a connection between the studied phenotype of pyrophosphate arthropathy and a locus located on the long arm of chromosome 8 (the symbol of the CCAL locus). A. G. Hughes et al. (1995) found a connection between the phenotype of primary chondrocalcinosis in a family from the UK and the CCAL1 locus, which is localized on the short arm of chromosome 5 in the 5p15 region. According to CJ Williams et al. (1996), the CCAL1 locus in members of an Argentine family with pyrophosphate arthropathy was located somewhat more proximally than in the previous case, in the 5p15.1 region. A similar genotype was found in members of a family from France.
Thus, the data from the described studies indicate that the familial form of pyrophosphate arthropathy is a clinically and genetically heterogeneous disease, which can be caused by mutations in at least three different genes.

