
All iLive content is medically reviewed or fact checked to ensure as much factual accuracy as possible.
We have strict sourcing guidelines and only link to reputable media sites, academic research institutions and, whenever possible, medically peer reviewed studies. Note that the numbers in parentheses ([1], [2], etc.) are clickable links to these studies.
If you feel that any of our content is inaccurate, out-of-date, or otherwise questionable, please select it and press Ctrl + Enter.
FDA Approves SetPoint Implant for Moderate to Severe Rheumatoid Arthritis
Last reviewed: 09.08.2025
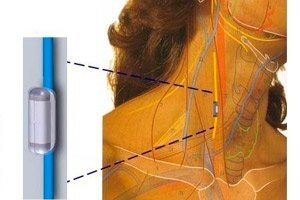
In some patients with rheumatoid arthritis (RA), biological and targeted synthetic DMARDs do not work perfectly or are poorly tolerated. Against this background, the SetPoint System, an implant for neuroimmune modulation via the vagus nerve, was approved in the United States.
What is approved?
The SetPoint System is an implantable device that delivers brief electrical stimulation to the vagus nerve once daily, activating innate anti-inflammatory and immune-restorative pathways. Indications: Adults with moderate-to-severe RA who are inadequately treated with or intolerant to advanced therapy (bio/tsDMARDs).
Evidence base (RESET-RA)
In the randomized RESET-RA study, 242 patients received either a real implant or a "dummy" device.
- Primary goal achieved: ACR20 by month 3.
- Plus, improvements in activity indicators and response rates up to 12 months of follow-up.
- 75% of participants were free of biologic/targeted synthetic DMARDs by 12 months.
- The procedure and therapy were well tolerated; serious adverse events were 1.7%.
Safety and convenience
The installation is a minimally invasive outpatient procedure. Once implanted, the device is programmed and automatically delivers stimulation on schedule, and is designed to last up to 10 years.
What does this mean for practice?
- A new class of RA therapy has emerged: neuroimmune modulation as an alternative/add-on to DMARDs.
- It is reasonable to consider in patients with insufficient response or intolerance to advanced regimens.
- Real-world clinical practice data and comparisons with active drugs for long-term efficacy/safety, as well as criteria for selecting responder patients, are needed.
Authors' comments
“The approval of SetPoint highlights the potential of neuroimmune modulation as a new approach to autoimmune diseases – harnessing neural pathways to combat inflammation,” said Mark Richardson, MD, PhD, lead investigator of RESET-RA (Harvard).
“Once implanted, the device automatically delivers therapy on a set schedule for up to 10 years, making life easier for people with RA,” he added.
