
All iLive content is medically reviewed or fact checked to ensure as much factual accuracy as possible.
We have strict sourcing guidelines and only link to reputable media sites, academic research institutions and, whenever possible, medically peer reviewed studies. Note that the numbers in parentheses ([1], [2], etc.) are clickable links to these studies.
If you feel that any of our content is inaccurate, out-of-date, or otherwise questionable, please select it and press Ctrl + Enter.
FDA approved Bimzelx for the treatment of hidradenitis
Last reviewed: 02.07.2025
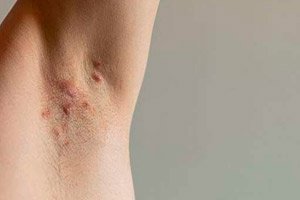 ">
">The U.S. Food and Drug Administration (FDA) has approved UCB's Bimzelx (bimekizumab-bkzx) to treat adults with moderate to severe hidradenitis suppurativa.
Bimzelx is the first and only approved drug that selectively inhibits interleukin 17F (IL-17F) in addition to interleukin 17A (IL-17A). This is the fifth indication for Bimzelx in the United States.
Basis for approval:
The approval is based on data from two Phase III clinical trials (BE HEARD I and BE HEARD II) conducted in adult patients with moderate to severe hidradenitis suppurativa.
Key performance indicator:
- HiSCR50: At week 16, a significantly greater proportion of patients treated with Bimzelx achieved ≥50% improvement in signs and symptoms of hidradenitis suppurativa (as measured by the Hidradenitis Suppurativa Clinical Response (HiSCR50) score) compared with placebo.
Secondary indicator:
- HiSCR75: The drug also provided clinically meaningful improvements in patients achieving ≥75% improvement (HiSCR75) at week 16, compared with placebo.
Sustainability of results:
- Clinical responses were maintained through week 48, with no new safety signals identified.
Expert opinion:
Dr. Alexa B. Kimball, BE HEARD program investigator and dermatologist at Beth Israel Deaconess Medical Center in Boston, said,
“The approval of Bimzelx for the treatment of moderate-to-severe hidradenitis suppurativa is an important development given the significant unmet clinical need and limited number of treatment options available today.”
